No e-bike, no electric car and no e-plane would be conceivable without this type of battery. However, there are a few strange things about this battery technology that you may not know. A short overview of unusual facts.
You can find them in your laptops, smartphones, drills or e-bikes: lithium-ion batteries. The super-batteries dominate in almost every kind of electrical device thanks to their long life and great weight-performance ratio. No wonder they are used not only in household appliances, but also in e-mobility.
But did you know that the batteries can be very dangerous? Or that the inventor of the technology died under mysterious circumstances? We have collected four unusual facts about lithium-ion batteries.
Dangerous: Lithium batteries can catch on fire by themselves
For all the many advantages the lithium-ion batteries offer, there is a crucial danger: they can overheat and catch fire.
The reason for this is in the accumulator itself: the electrolyte solution in the accumulators is usually combustible. In many cases, this has already caused fires. The Japanese manufacturer Panasonic had to recall 43,000 laptop batteries three years ago. The reason: potential fire hazard.
But one of the most spectacular examples of the fire dangers of the batteries was Boeing. Due to the high temperature and pressure differences, a lithium battery caught fire on an airplane. The aircraft builder had to issue a worldwide flight ban on all 50 of its 787 Dreamliners.
To prevent such fires in the future, scientists from Forschungszentrum Jülich have developed a battery with a solid electrolyte. This is to prevent leakage of the electrolyte and stop the fire hazard.
Infected: If batteries are treated with viruses, they last longer
With electric batteries, mass and reach are inversely related. The lighter the batteries are, the further an electric car can drive. The search for the ultimate lithium-ion batteries has long since become an international race.
Material researchers at the Massachusetts Institute of Technology recently came up with the unusual idea of infecting lithium-oxygen batteries with viruses. The viruses which the researchers used began to build thin wires in the batteries. This increases the performance of the memory.
Wasting away: Batteries lose their life as soon as they leave the factory
Of course we all know that lithium batteries do not last forever. But did you know that the wear om the batteries starts even before the first use?
Of course, the actual life of the batteries depends strongly on the internal charge, the usage as well as on the outside temperature and some other factors. But the creeping death begins at the moment when the lithium-ion batteries leave their production plant.
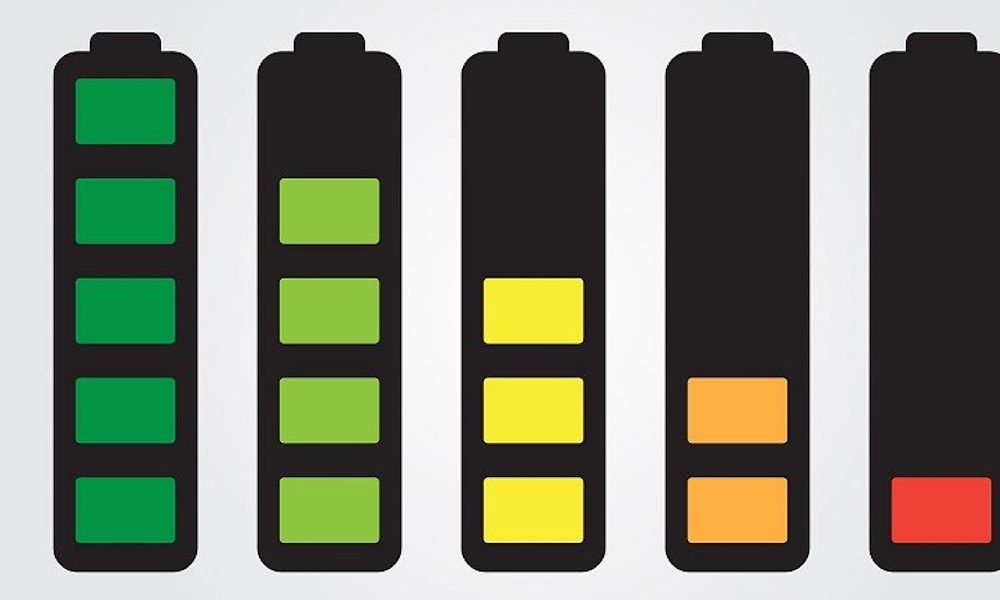
Every year after the production of a lithium battery, its storage capacity is reduced by 20 percent.
This also means you should look at the year of manufacture when buying used (or very cheap) batteries. And: if you get a replacement battery, you should not wait to use it until the first battery has lost all its power, but instead. alternate between using the old battery and the replacement.
Tragically, inventor of the lithium battery died under mysterious circumstances
Gilbert Newton Lewis is considered the father of lithium-ion batteries. Lewis taught chemistry at the University of California, Berkeley. In 1912 he developed the storage technology we now know as lithium-ion batteries. But Lewis never received the top prize for his great invention: he was nominated for the Nobel Prize in chemistry 41 times, but never won.
All the more tragic was his death. He was found dead in his laboratory in Berkeley by a student in 1946. The official cause of death was “coronary heart disease” caused by a laboratory accident.
According to rumors, he had lunch with his greatest rival, Irving Langmuir, on the day of his death. Langmuir had received a Nobel Prize in 1932, an honor for which Lewis was skipped over. Some suspect, therefore, that after the meeting, Lewis was so depressed that he committed suicide.